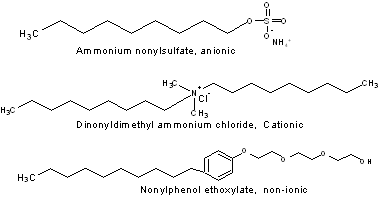
Surfactant Types
Most surfactants have two distinguishing features: they have a “polar” head and a long, non-polar tail. The polar end or head can be one of three different types: Anionic, cationic, or non-ionic. The surfactants that have exhibited viscosity reduction in sugar propellant formulations are of the anionic type. The following cartoons represent some of these surfactant types.
Surfactants in sugar propellant formulations
The following link (surfactant data - A) connects to a spread sheet listing the various anionic surfactants that have been evaluated for viscosity reduction in a standard 65/35 weight ratio KNO3/sorbitol propellant formulation. Many surfactants, especially the ionic ones, are supplied as solutions in water or alcohol. The actual surfactant level added to the formulation is listed in the spread sheet and not the percent surfactant solution added. Thus if 1% of a 50% surfactant in water solution was added to a propellant formulation the actual level of surfactant is 0.5%. Since, in some cases, a small amount of water is being added to the formulation along with the surfactant, an experiment was undertaken to make sure that viscosity reduction was being accomplished by the surfactant and not the water. 1% water was added to a standard KNSO formulation and mixed while hot. No viscosity reduction was observed. In most cases those surfactants which did exhibit a viscosity reducing effect proved to be more effective if used in greater amounts. Unfortunately this effect was offset by lowering the surfactant containing formulation’s ability to ignite at atmospheric pressure.
Updates to this table will be made when new surfactant types are obtained. Surfactant data - B lists those non-ionic surfactants that have been evaluated for viscosity reduction. A spreadsheet on the evaluation of various surfactants with other types of sugars will also be added once these experiments have been made. Also anticipated at some point will be the evaluation of surfactants with other oxidants such as sodium or calcium nitrate, etc.
Many of the surfactants evaluated in this study were obtained as free samples from The Stepan Company (<http://www.stepan.com/en/>). Several off-the-shelf surfactants work reasonably well as viscosity reducers. Shampoo formulations listing the ammonium or sodium salts of laureth sulfate as the first ingredient work quite well. The laureth rather than the lauryl sulfates seem to work better. The lauryl group is the long chain non-polar group of the surfactant. A lauryl group contains an unbranched string of 12 carbon atoms. Evaluation of various surfactants seems to indicate that the most effective viscosity reducers are those surfactants which contain an effective carbon atom string of 10 or fewer carbons. Further work is planned to substantiate this theory.